The manufacture of sulphuric acid is called Contact Process.
The raw materials required in contact process are sulphur, air and water.
The contact process involves THREE stages of chemical reactions as below:
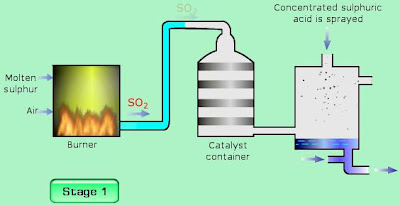
Stage I : Production sulphur dioxide, SO2
Sulfur is burnt in dry air in the furnace to produce sulphur dioxide.
S + O2 → SO2

Stage II : Conversion of SO2 to SO3
Pure SO2 reacts with oxygen in catalytic converter to produce sulphur trioxide at a temperature between 450°C to 550°C.
2SO2 + O2 → 2SO3
This stage requires catalyst vanadium(V) oxide.

Stage III : Production of sulphuric acid
In the absorber, sulphur trioxide is dissolved in concentrated sulphuric acid to produce oleum, H2S2O7.
H2SO4 + SO3 → H2S2O7
Oleum is the diluted in water to produce two molecule of sulphuric acid.
H2S2O7 + H2O → 2H2SO4
SO3 gas is not directly dissolved in water to produce H2SO4 because the reaction releases large amount of heat and is able to vaporize sulphuric acid and produces acid mist.
Among the uses of sulphuric acids are:
i. to manufacture fertilizers
ii. to manufacture detergent
iii. to manufacture paint pigment
iv. to remove metal oxide from metal surface
v. to manufacture pesticide
vi. as the electrolyte in lead-acid accumulators
The burning of products manufactured from sulphuric acid will produce SO2. The SO2 dissolved in atmospheric water vapour to produce acid and subsequently causes the acid rain.
SO2 + H2O → H2SO3








